
Iron, atomic structure Stock Image C018/3707 Science Photo Library
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Bohr Model Model Atomic Iron Atomic Orbital PNG, Clipart, Area, Atom
Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr's model was taken seriously, despite the many assumptions that Bohr needed to derive it. Figure \(\PageIndex{1}\): Quantum numbers and energy levels in a hydrogen atom.
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
Iron Periodic Table Electrons Review Home Decor
Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately dropping back to a lower energy level and emitting the energy difference between the two energy levels. The existence of the atomic spectra is support for Bohr's model of the atom.

Iron Atom Structure Stock Vector Art & More Images of 2015 493163650
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.

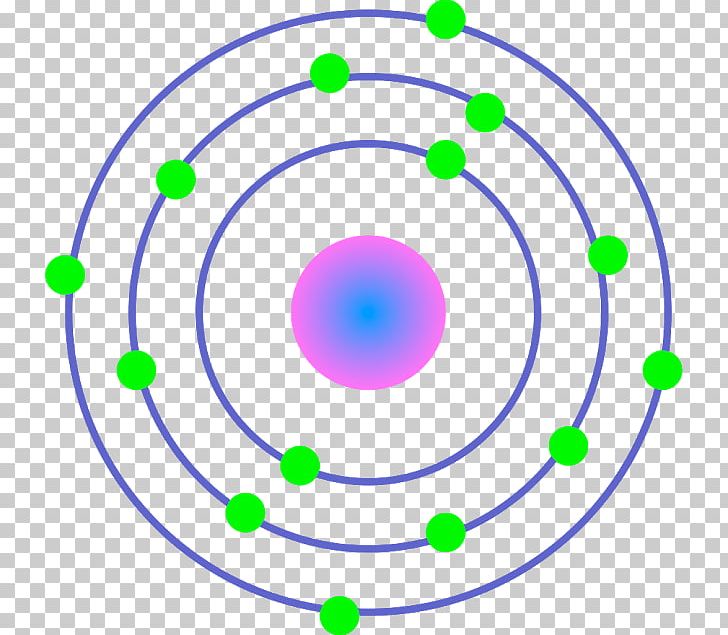
Iron (Fe) Bohr Model
The electron cloud model An electron cloud model of a helium-4 atom is shown below. [What do the scales mean on this model?] In this model, the black "cloud" represents the volume of space where electrons are likely to be found. The darker the region, the more likely electrons are to be found there.
How To Draw Bohr Diagrams Images and Photos finder
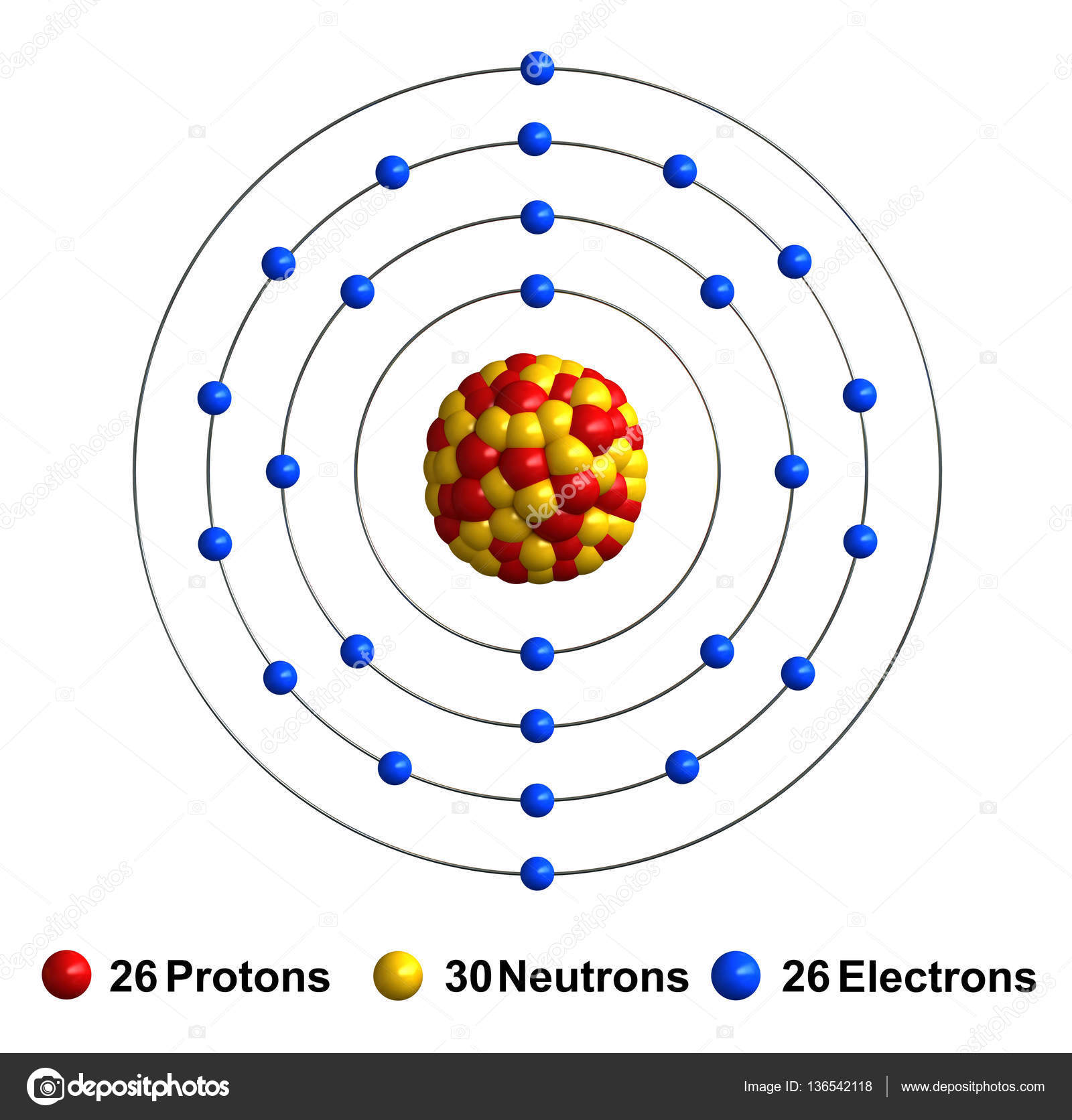
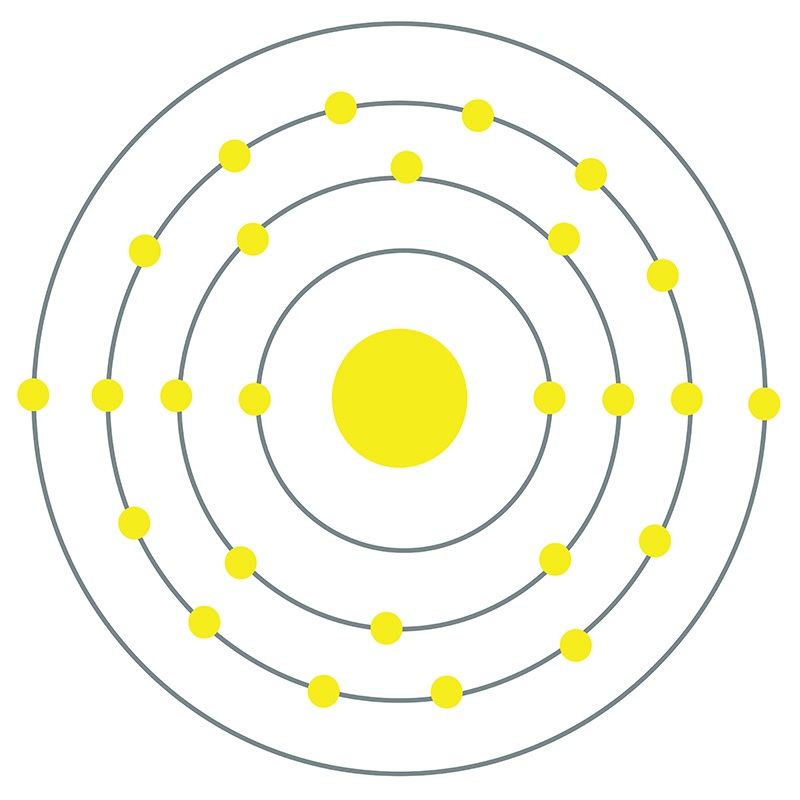
The Bohr Model of Iron (Fe) has a nucleus that contains 30 neutrons and 26 protons. This nucleus is surrounded by four electron shells namely K-shell, L-shell, M-shell, and N-shell. The 1 st shell has 2 electrons, the 2 nd shell has 8 electrons, the 3 rd shell has 14 electrons and the 4 th shell has 2 electrons. Page Contents show
3d render of atom structure of iron Stock Photo by ©oorka5 136542118
Iron Bohr model December 14, 2023 by Deep The information on this page is fact-checked. Iron Bohr model In the iron Bohr model, the nucleus comprises 26 protons and 30 neutrons. Surrounding this nucleus are four electron shells, accommodating a total of 26 electrons.

Iron atom Bohr model stock vector. Illustration of isolated 267662069
Iron (Fe) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Iron atom electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.

Arriba 63+ imagen hierro modelo de bohr Abzlocal.mx
The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). Bohr used the term energy levels (or shells) to describe these orbits of differing energy. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another.

5 Cool 3d Model Of Silicon Atom Wanted Mockup
Basic Information Name: Iron Symbol: Fe Atomic Number: 26 Atomic Mass: 55.845 amu Melting Point: 1535.0 °C (1808.15 K, 2795.0 °F) Boiling Point: 2750.0 °C (3023.15 K, 4982.0 °F) Number of Protons/Electrons: 26 Number of Neutrons: 30 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 7.86 g/cm 3 Color: Silvery

Periodic Network 2012 [licensed for use only] / Iron
The Bohr model, introduced by Danish physicist Niels Bohr in 1913, was a key step on the journey to understand atoms. Ancient Greek thinkers already believed that matter was composed of tiny.
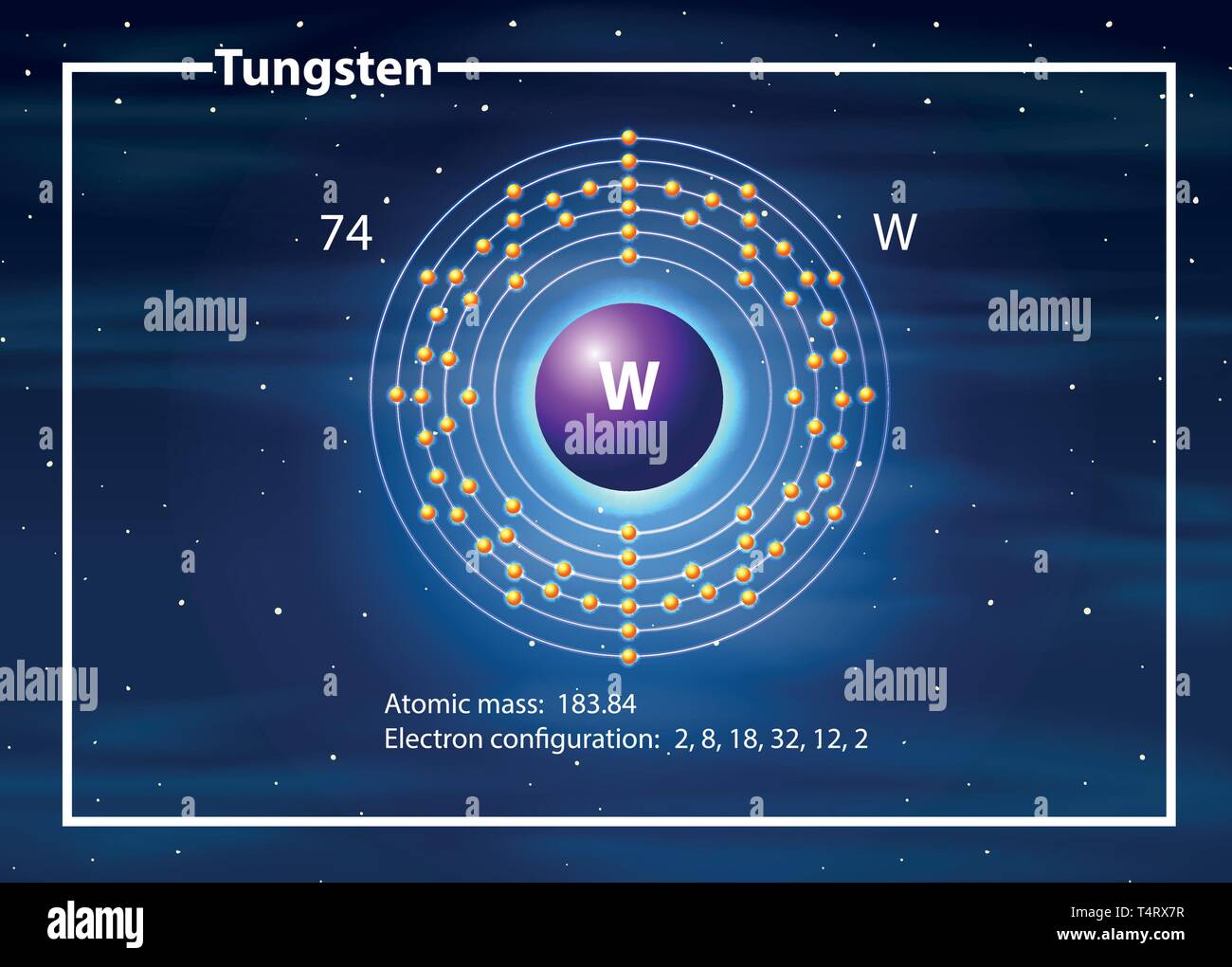
Tungsten atom diagram concept illustration Stock Vector Image & Art Alamy
Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which is closer to nucleus has less energy Postulates of Bohr's Model of an Atom
Bohr Model of the Atom Overview and Examples
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.

Iron(III) Chloride AMERICAN ELEMENTS ® / Ferric Chloride
The Bohr Model for Hydrogen (and other one-electron systems) In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum.. iron, and carbon, along with smaller amounts of other elements. During the solar eclipse of 1868, the.
Iron (Fe) AMERICAN ELEMENTS
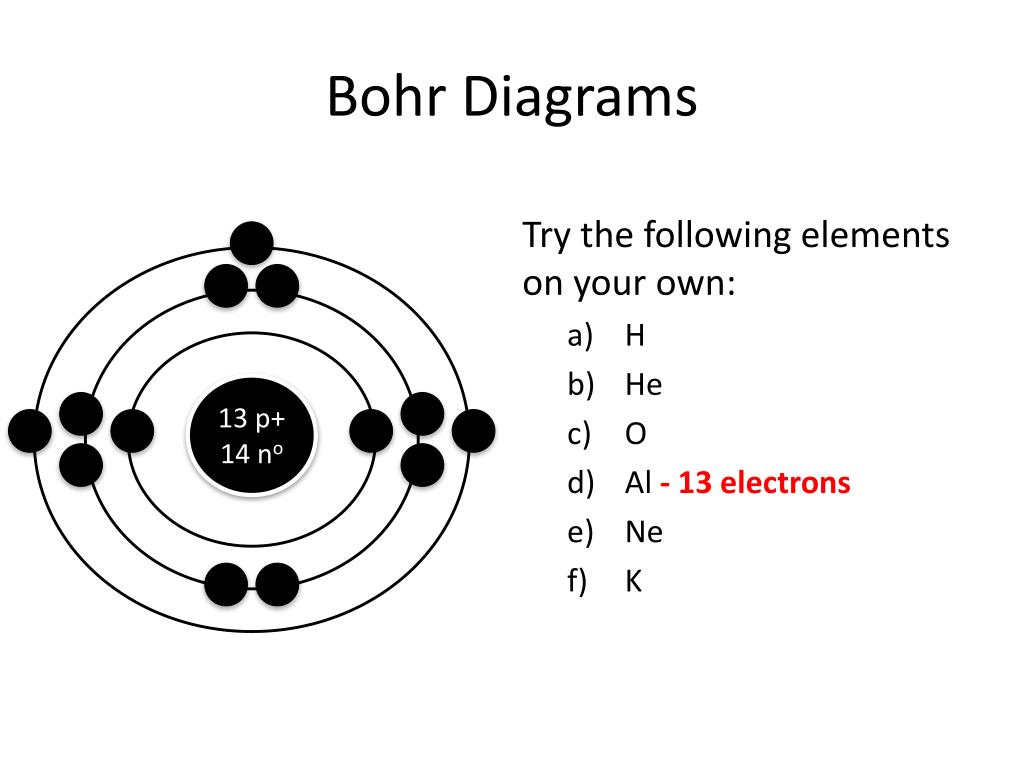
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Belajar 5 Teori Model Atom Dan Penjelasannya, Menarik Untuk Di Simak
The Bohr model describes the structure of an atom as a central nucleus containing protons and neutrons, with electrons orbiting in specific energy levels around it. Electrons can jump between these energy levels by absorbing or emitting energy. This model helps us understand basic atomic properties and electron behavior in atoms. K Shell